About Galafold
WHAT IS
GALAFOLD?
Galafold (migalastat) is the first in class, oral pharmacological chaperone1 approved for long-term treatment of adults and adolescents aged 12 years and older with a confirmed diagnosis of Fabry disease and who have an amenable mutation.2
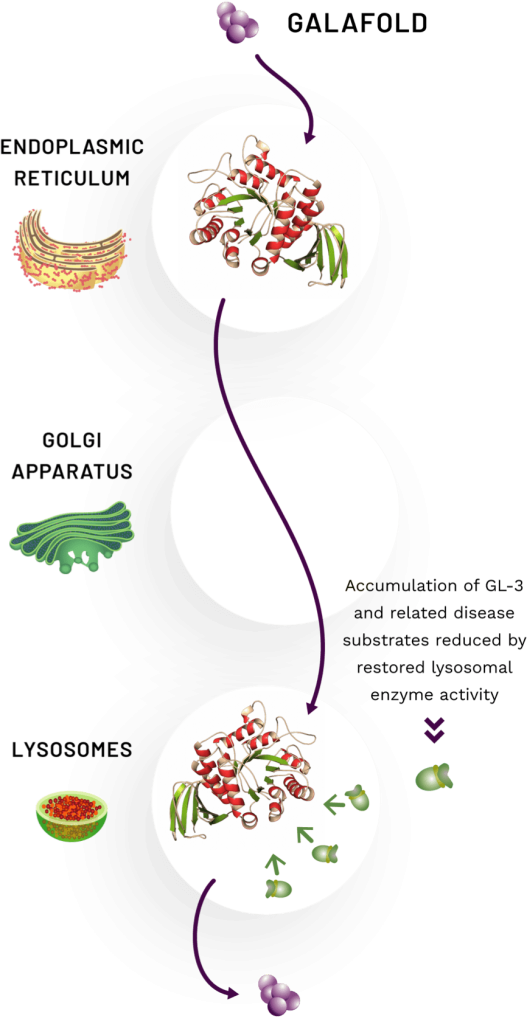
HOW DOES IT WORK?
Galafold is a first in class1 oral pharmacological chaperone that selectively and reversibly binds to the body’s own dysfunctional enzyme, stabilising it and facilitating its proper trafficking to the lysosome.1 Once within the lysosome, dissociation of Galafold restores lysosomal activity of the enzyme α-galactosidase A (α-Gal A), leading to reduction in disease substrates.2
The proposed mechanism of action of Galafold is shown in the image and is as follows:
- The stabilised α-Gal A enzyme with bound Galafold leaves the endoplasmic reticulum (ER)3
- Once the stabilised enzyme with bound Galafold reaches the lysosome, Galafold dissociates from the α-Gal A enzyme2
- The released enzyme is freed to catabolise GL-3 and related disease substrates2
- Stored substrates are believed to displace Galafold and take over the stabilisation of the mutant enzyme4
- Galafold leaves the lysosome and is excreted from the body via urine2
Galafold has demonstrated efficacy in three Phase 3 trials, an open label extension study and an evaluation of real-world evidence.1,2,5,8
This animation outlines the impact of Galafold on renal function in people with Fabry disease.
Amenability
Galafold is indicated for long-term treatment of adults and adolescents aged 12 years and older with a confirmed diagnosis of Fabry disease and who have an amenable mutation.2 As there have been a significant number of mutations identified to date,2 it is essential that amenable mutations can be accurately identified so that the relevant patients with a confirmed diagnosis of Fabry disease may be treated. Identifying which GLA mutations are amenable to Galafold is determined using the Galafold Amenability Assay.
Amenable GLA mutations are those expected to result in α-Gal A enzymes that can be bound and stabilised by Galafold, resulting in an increase in α-Gal A activity above prespecified criteria measured in the in vitro Galafold Amenability Assay.6 The amenability assay, which is carried out by an independent, third-party clinical laboratory, is not intended to be used to classify mutations as pathogenic or aid in the diagnosis of Fabry disease.6 Watch this video to learn more about Galafold amenability assay test.
The validation of pharmacogenetics for the identification of Fabry patients to be treated with migalastat
Amenability Criteria Defined
Amenability requires both an absolute increase in α-Gal A activity ≥3.0% of wild type and a relative increase ≥1.2 fold over baseline.
An estimated 35% to 50% of patients have GLA mutations amenable to Galafold.1
See the Galafold® Summary of Product Characteristics for a list of amenable mutations.
Development of the GLP-validated Galafold Amenability Assay
| Measuring Enzyme Activity Both absolute and relative increases in α-Gal A activity are analysed | To be amenable, a mutation must meet criteria for both absolute and relative increases in α-Gal A activity. This is designed to ensure both clinically relevant (absolute increase) and sufficiently robust (relative increase) increases in enzyme activity.6 |
| Amenability Criteria Defined Amenability requires both an absolute increase ≥3.0% of wild type and a relative increase ≥1.2 fold over baseline | Potential criteria were compared against an in vivo assay in males to determine which amenability criteria were most predictive of clinical response. The selected amenability criteria had the highest positive predictive value of 1.0 and a negative predictive value of 0.875. Sensitivity and specificity of the assay are moderately lower in females, reflecting the difficulty of measuring response of the mutant enzyme in the presence of wild-type α-Gal A from the second GLA allele. It does not necessarily indicate lower potential for clinical benefit.6 |
| Migalastat Concentration used In Assay A concentration of 10 µmol/L migalastat was selected | The in vitro migalastat concentration of 10 µmol/L corresponds to the approximate mean maximum plasma concentration (Cmax) in patients with Fabry disease after single oral dose of 150 mg migalastat hydrochloride.6 |
| Assay Validated Assay was validated using data from Phase 2 and Phase 3 clinical studies | Amenability criteria were further confirmed based on pharmacodynamic response to Galafold in Phase 2 and Phase 3 studies. The comparison showed high specificity, sensitivity, and positive and negative predictive values.6 |
| Additional Mutations Categorised Mutations in clinical study patients are representative of all mutations amenable to Galafold | Amenable mutations (n=51) from patients in clinical trials were compared to all 348 known amenable mutations as of 2018.7 The 51 amenable mutations included in clinical studies are representative of the larger set of all amenable mutations identified.7 |
Characteristics of the Galafold Amenability Assay

No blood sample or DNA swab is required6

Testing of each mutation is replicated with 20-fold redundancy6

The assay is performed by an independent, third party clinical laboratory6
References
- Hughes DA, Nicholls K, Shankar SP, et al. Oral pharmacological chaperone migalastat compared with enzyme replacement therapy in Fabry disease: 18-month results from the randomised phase III ATTRACT study. J Med Genet. 2017;54(4):288-296.
- Summary of product characteristics. Galafold 123 mg hard capsule. Amicus Therapeutics Ltd. Available at: https://www.emcpi.com/pi/37288
- Germain DP. Fabry disease. Orphanet J Rare Dis. 2010;5:30.
- Sánchez-Fernández EM, García Fernández JM, Ortiz Mellet C. Glycomimetic-based pharmacological chaperones for lysosomal storage disorders: lessons from Gaucher, GM1-gangliosidosis and Fabry diseases. Chem Commun. 2016;52(32):5497-5515.
- Germain DP, Hughes DA, Nicholls K, et al. Treatment of Fabry’s disease with the pharmacologic chaperone migalastat. N Engl J Med. 2016;375(6):545-555.
- Benjamin ER, Della Valle MC, Wu X, et al. The validation of pharmacogenetics for the identification of Fabry patients to be treated with migalastat. Genet Med. 2017;19(4):430-438 (and Supplementary Appendix).
- Wu X, Williams H, Bond S, Bronfin B, Benjamin ER. Migalastat amenability assay comparison between amenable mutations studied in clinical trials and the broader population of all amenable mutations. Presented at the 2018 American College of Medical Genetics and Genomics (ACMG) Clinical Genetics Meeting; April 10-14, 2018; Charlotte, NC.
- Hughes DA, Sunder-Plassmann G, Jovanovic A, et al. Renal and multisystem effectiveness of 3.9 years of migalastat in a global real-world cohort: results from the followME Fabry Pathfinders registry. J Inherit Metab Dis. 2024;1-14. doi:10.1002/jimd.12771. Online ahead of print.
